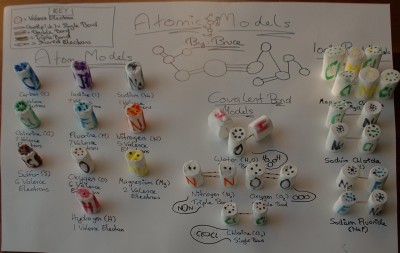
A science project I had to do in class about atomic molecules. It shows the different elements on the left side (including carbon, oxygen, nitrogen, sodium, and hydrogen) with their valence electrons shown as black dots on top. The actual molecules (in the middle and on the right side) are shown with the atoms as marshmallows and the bonds formed as toothpicks. In the middle are covalently bonded molecules (formed when atoms share valence electrons to get a total of eight), and the examples are water (H2O), Nitrogen (N2), Oxygen (O2) and Chlorine (Cl2). On the right are molecules with ionic bonds holding them together (which occur when atoms take or give away valence electrons to get a total of eight), and the examples are magnesium chloride (MgCl2), sodium chloride (also known as table salt; NaCl), and sodium fluoride (NaF). This project was an interesting way of showing how the bonds in molecules work, and overall I had fun with it.
Our adventures in the Pacific Northwest
